Conduct 7 Sweet Candy Experiments

Candy isn’t just a sugary treat. You can learn something by using it in some fun science experiments.
DIVING CANDY
Can you make a piece of candy dive and then surface again?
 Time: 5 minutes
Time: 5 minutes
Skill Level: Easy
WHAT YOU NEED
- Warheads (recommended) or other candy that is sealed in plastic, such as a Gobstopper or striped mint. Do not unwrap! The candy wrapper must be sealed on both edges, not just twisted tight.
- A plastic bottle with a tight-fitting lid, filled to the brim with water. (Soda bottles work better than juice or syrup bottles.)
- Water
- Bowl (optional)
- Pin (optional)
- Needle-nose pliers (optional)
WHAT TO DO
1. Drop the wrapped candy into the bottle of water. (If you’re using a bottle with a narrow neck, you might have to gently push the candy through the opening.) Does the candy float?
2. If the candy floats, tightly fasten the lid of the bottle.
3. Squeeze the bottle. Does the candy sink? (Hint: Small hands might have a hard time squeezing the bottle hard enough to make the candy dive. To try another method, lay the bottle down sideways on the floor and stand on it.)
4. If the candy does not sink, try one of these fixes:
- Using needle-nose pliers, pull the candy out of the bottle. Use a pin to poke a tiny hole in the wrapper, near an edge or the bottom. Put the candy back in the bottle, with the hole on the bottom side. The wrapper should slowly start to fill with water, trapping an air bubble at the top of the wrapper.
- Try a different piece of candy.
- Try a different bottle, such as a small soda bottle that is easy to squeeze.
WHAT’S HAPPENING
Most candy is denser than water, so it sinks. But if air is trapped inside the wrapper, the wrapped candy floats.
To float, a piece of candy has to push aside more than its weight in water. Candy is denser (heavier) than water, so it sinks. But if a piece of candy is in a wrapper filled with air, it is lighter than the equivalent volume of water, and it floats.
When you squeeze the bottle, the water squeezes the candy. The air bubble trapped inside the wrapper gets smaller. The candy pushes aside less water, and the water no longer supports it. The candy sinks. When you stop pushing the bottle, the air bubble expands (gets bigger). The wrapper pushes aside more water, and the candy floats.
Some fish have a swim bladder that functions the same way. When they contract the air-filled bladder, the fish descend. When they let the air sac expand, they rise. Other fish adjust their buoyancy by letting air in and out of the swim bladder, making it bigger or smaller. This helps the fish swim up and down.
Note: Since every piece of candy is wrapped differently, some of them will not float, and some of them will not sink. You might have to try several pieces of candy to get the right air bubble.

CAN YOU TASTE PH?
What can you use to test acidity: pH paper or your tongue? Or both?
Time: Up to an hour
Skill Level: Medium
WHAT YOU NEED
- A variety of sour candies such as Warheads, Lemonheads, Sour Patch Kids or Skittles
- Small bowls
- pH indicator paper, ranging from a pH of about 1 to 7
- Warm water
WHAT TO DO
1. Put a few pieces of candy in each bowl. (Use a separate bowl for each kind of candy.)
2. Pour warm water into each bowl until the candy is covered.
3. Let the sour part of the candy dissolve. (Some candies have a sour shell, and some need to dissolve completely.)
4. Taste a spoonful of water from each bowl. How sour is it?
5. Line the bowls up in order of sourness.
6. Test each bowl of water with a pH indicator strip. Do the results match the results of your tasting?
WHAT’S HAPPENING
Sour taste is caused by acid, so the more sour your candy is, the more acid it contains. You should be able to test this both with pH paper and with your tongue.
If your taste test doesn’t exactly match the pH paper test, there are a few possible explanations. Sometimes it’s hard to tell exactly what result the pH indicator paper gives, especially when you’re using it to test colored candy. Also, some flavors might cover up or distract you from acidic tastes.
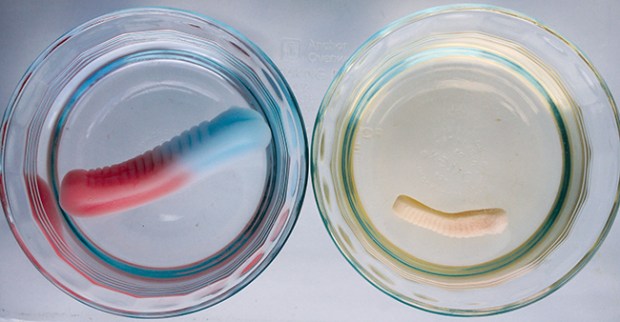
SALTWATER GUMMI SOAK
Time: 2 days
Skill Level: Medium
WHAT YOU NEED
- 4 gummi worms or other gummi candy
- 3 small clear bowls with about 1 cup of water each
- Sugar
- Salt
WHAT TO DO
1. Add a spoonful of sugar to one bowl of water.
2. Add a spoonful of salt to another bowl of water.
3. Put a gummi candy in each bowl and set the extra gummi bear aside for comparison.
4. After several hours, check the gummi candies to see what size they are. Which ones have changed the most?
WHAT’S HAPPENING
A cup of fresh water is a dilute solution. When you put salt in water, you make a concentrated solution. The more salt, the more concentrated the solution is. If water can pass from a dilute solution into a concentrated one, it will. Nature is trying to make the two solutions equally concentrated. This process is called osmosis.
You can see osmosis in action when you put a gummi worm in fresh water. Water flows into the gummi worm, diluting the sugary gelatin mix (a concentrated solution). The gummi worm expands. But if you put a gummi worm in salt water, the salt water is already concentrated. Not as much water is needed to dilute the gummi worm, so the gummi worm will expand less. In fact, depending on how concentrated your solution is, it might not expand at all.
Osmosis is also the process that draws water into plant roots and up where it’s needed. If you watered plants with salt water, the plant cells wouldn’t be able to absorb any water, just like gummi bears.
POP ROCKS DENSITY LAYERS
If you pour together colored solutions of different densities, you can make density layers. Can you make density layers without pouring?
 Time: 20 minutes
Time: 20 minutes
Skill level: Medium
WHAT YOU NEED
- 3 packages of Pop Rocks (two packages of one color, one package of a different color)
- Narrow glass filled with water
WHAT TO DO
1. Pour 2 packages of the same color Pop Rocks into the glass of water. Wait several minutes until the Pop Rocks dissolve.
2. Pour the other package of colored Pop Rocks into the water. Do they mix with the colored water or float on top?
WHAT’S HAPPENING
A less-dense liquid will float on top of a denser liquid. (This is why oil floats on water.)
When you dissolve the first two packages of Pop Rocks, you create a dense sugar solution at the bottom of the cup. When you add the third package, the Pop Rocks float on top of that dense solution before they dissolve to create a stripe of color.

WARTY LICORICE
Can you make a smooth piece of licorice grow warts?
Time: 5 minutes
Skill Level: Get a grown-up
WHAT YOU NEED
- Twizzlers licorice twists (the Pull-n-Peel variety works especially well)
- Microwave-safe plate
- Microwave (Alternative: baking sheet lined with aluminum foil or parchment paper, and oven)
WHAT TO DO
1. Place the licorice on the plate.
2. Microwave on low or medium heat, checking it every 30 seconds so it doesn’t start to burn. Does your licorice grow warts?
Alternative: Place licorice on baking sheet and heat in 300°F oven. Check back every 5 minutes until you see warts growing.
WHAT’S HAPPENING
Licorice, like most soft candy, contains a little water. The warts might be created by water making tiny pockets of steam when it’s heated.
Why doesn’t licorice melt in the oven or microwave? Because licorice contains flour, which doesn’t melt. This also means that by some definitions, licorice isn’t actually candy!
SWIMMING GUMMI FROG
Can you make a gummi frog swim?
 Time: 2 days
Time: 2 days
Skill Level: Easy
WHAT YOU NEED
- Gummi candy frog with a marshmallow coating on the bottom, such as a Haribo brand frog
- Clear dish of water
WHAT TO DO
1. Drop the frog into the dish of water.
2. Wait 2 days. What happens to the frog? What can you see in the marshmallow?
WHAT’S HAPPENING
As the gummi frog absorbs water, several things start to happen. The frog absorbs water and expands. As it does, its density changes, becoming closer to the water it is absorbing. It also stretches out the marshmallow, expanding the air bubbles. (Look closely, and you can actually see the air bubbles.) It might also be losing heavy sugar, since sugar dissolves into the surrounding water.
As the frog absorbs water, its density (weight and volume) becomes closer to that of the water surrounding it. If the air bubbles underneath get big enough, there’s just enough change in density to gently lift the gummi frog off of the bottom of the bowl.

TRANSLUCENT TAFFY
Can you turn taffy translucent?
Time: 30 Minutes
Skill Level: Get a grown-up
WHAT YOU NEED
- Saltwater taffy, Laffy Taffy or other opaque (not translucent) fruity chew
- Foil-lined baking sheet
- Oven
WHAT TO DO
1. With an adult’s help, preheat the oven to 300°F.
2. Place the unwrapped candy on the baking sheet.
3. Heat the candy in the oven for up to 30 minutes, checking every few minutes. What does the candy look like?
WHAT’S HAPPENING
Have you ever watched a taffy-pulling machine? Metal arms stretch and fold the soft candy, stretching and folding it over and over. As this happens, tiny air bubbles get trapped in the taffy, making it soft and chewy. (Without the air bubbles to separate the sugar molecules, the taffy would be as hard as a lollipop.)
The air bubbles also break up light rays when they pass through. Instead of passing right through the candy, the light gets absorbed or refracted by the air bubbles, making the candy look cloudy. When you melt it into a thin puddle, and some of the air bubbles escape, the taffy becomes translucent again.
Experiment Tips
- Don’t eat or drink the experiments. Candy might have germs on it if people have touched and handled it.
- Wear an apron or an old T-shirt, because candy and oil can stain clothes.
- Use tap water or room-temperature water unless the instructions say otherwise.
- Always ask a grown-up to help you heat candy in the microwave or the oven. Melted candy can get hotter than boiling water. If you touch it, you could get burned.
- Microwave times will depend on what kind of microwave you use. Always watch candy in the microwave to make sure that you don’t heat it too much.
- When heating candy in the oven, line your baking sheet with foil or parchment paper. This will save you from scrubbing sticky messes!
- Keep wet rags or paper towels ready to clean up sticky spills.
- If you leave candy water sitting around for several days, it might grow mold. If this happens, throw the experiment away and start over.
 From Candy Experiments 2 by Loralee Leavitt (Andrews McMeel Publishing LLC, $14.99) candyexperiments.com
From Candy Experiments 2 by Loralee Leavitt (Andrews McMeel Publishing LLC, $14.99) candyexperiments.com
are these even good experiments???
try putting sour patch kids in different liquids
heres another one! Coke+Mentos=BOOM!
Hint: Take this one outside, please.
i like the gummi soak
cool
I put a gummi bear in water and even after 2 days nothing happened -.-
Yeh Right
Cool.
Try putting mentos in coke you’ll get an soda explosion😀
It didn’t help me at all
facts
Cool
Meow I’m a cat
this is great
This is really cool can you please put some more cool things
is gummy worms edible after sugar and water